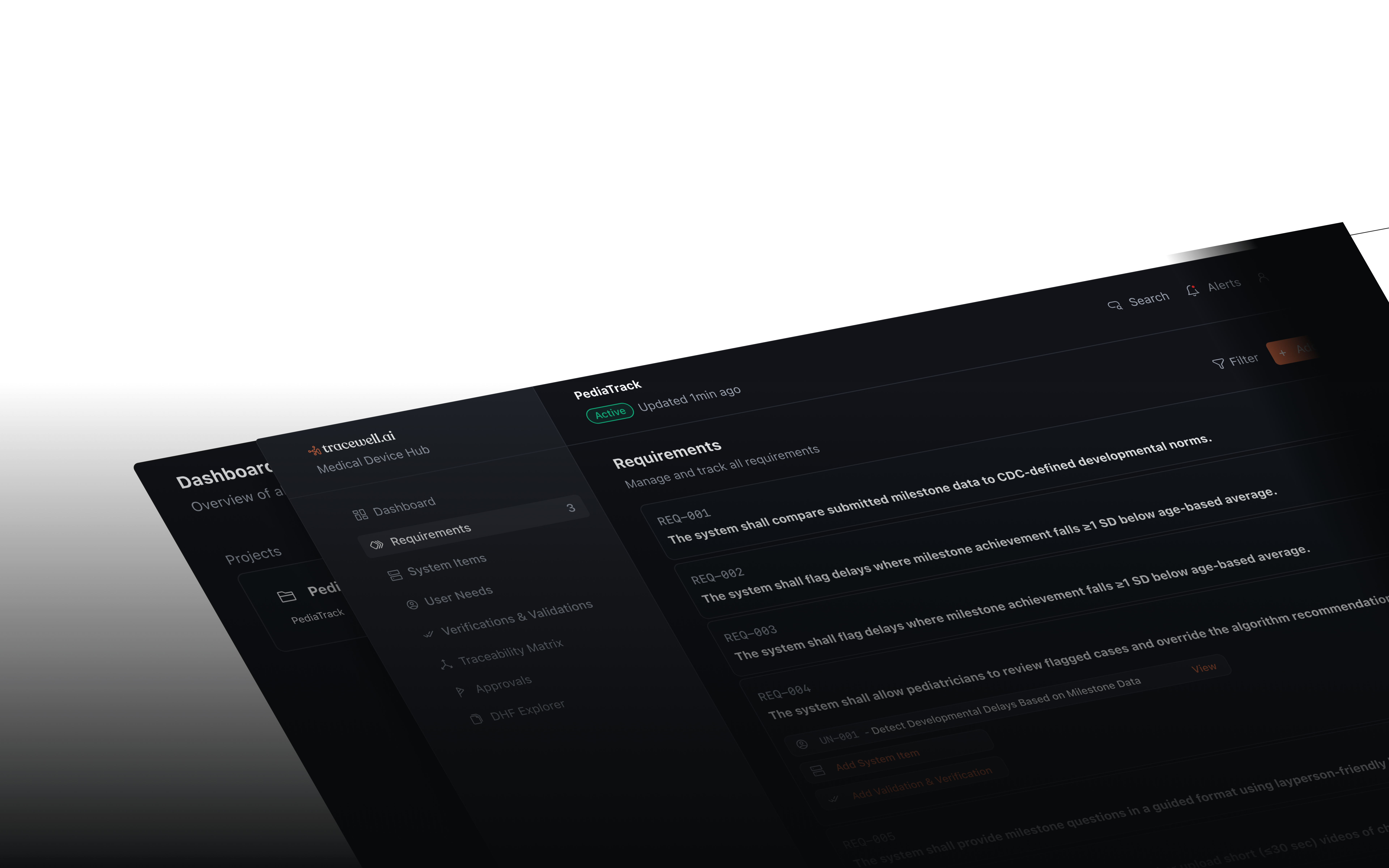
Agile Compliance for Modern SaMD Teams
Accelerate your path to FDA-ready documentation. Extract Design History Files from GitHub, Jira, and Linear automatically.
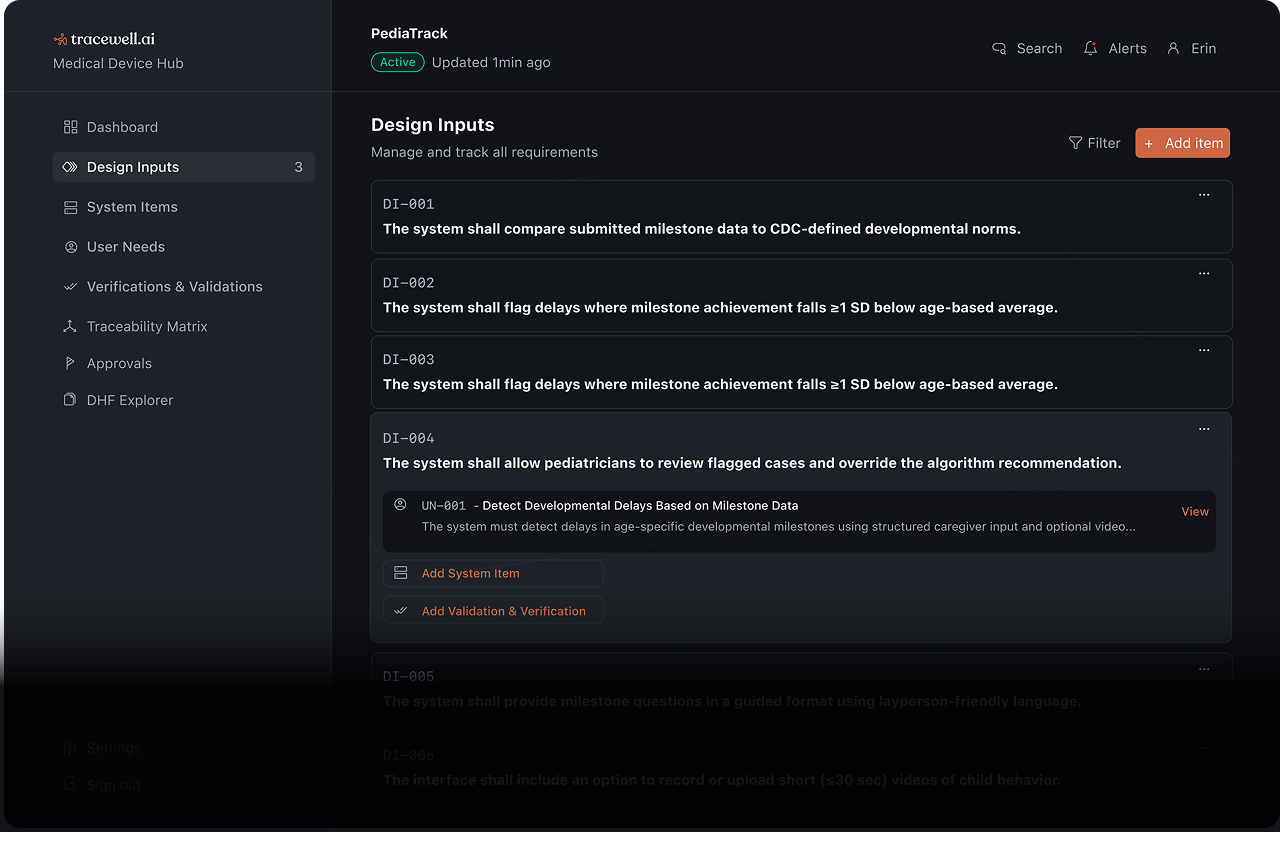
Your team already builds quality software. We help you document it for the FDA.
Keep Your Workflow
Work in the tools you know. No new systems to learn.
Bridge Teams
Connect development work to regulatory requirements automatically
Prevent Rework
Catch compliance gaps early, before regulatory review
No More Context Switching
Engineers code in GitHub
Product teams manage in Jira/Linear
Designers create in Figma
Regulatory gets automated DHF
Everyone stays in their workflow
Built for Cross-Functional Teams
Real-time visibility for regulatory teams
Product requirements become compliance documentation
Design decisions map to risk assessments
Engineering artifacts connect to design outputs
Frequently Asked Questions
Agile development.
Compliant delivery.
Built from successful FDA submission experience
Tracewell AI helps generate documentation aligned with regulatory frameworks like ISO 13485 and FDA 510(k) submissions. It is not a substitute for legal, regulatory, or quality system advice.